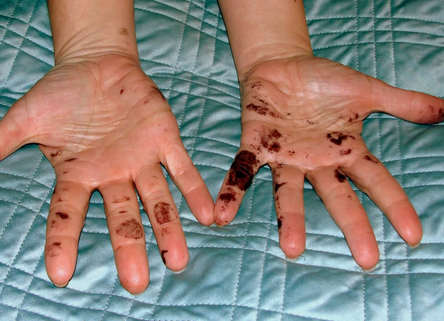
 My students and I were working on an acid/base lab when I went on a "clean all the glassware" spree. Unfortunately I had a flask under my hood that I grabbed in a very "hurricane-ish" way and this is the result. The chemical was silver nitrate and it permanently stains your hands, and remains there until the cells replensish themselves. It was a short 3 weeks ago and only my super dark pinkie remains stained. The moral of the story? Don't save the silver nitrate in solution in your hood!  Phew. I just finished a lab with my students which involved copper wire and silver nitrate. NORMALLY, I have zero tolerance for "tom-foolery" in lab, however...this was clever and harmless goofiness. The lab involves placing copper wire in a solution of silver nitrate and observing the single replacement reaction as it unfolds. A precipitate (the silver) attaches itself to the wire and it looks quite a bit like a pipe cleaner in it's earliest stages. One of my students called me from across the room, with an edge of concern in his voice. I said, "what the..." as I approached and saw the neon glow coming from one of their test tubes (with H20). What are the odds that a high school junior would have an actual neon colored pipe cleaner in his backpack? I LOVE TEACHING HIGH SCHOOL STUDENTS...
|
Julie and JenavieveA geeky mother and daughter working to bring science and art together. To get to know us better, check out our about page! Archives
January 2017
Categories
All
|






 RSS Feed
RSS Feed
